Although the atomic theory proposed by John Dalton created a basic structure of the atom, the general idea of molecules was not cleared. In 1809, Frech chemist Joseph-Louis Gay-Lussac and others began doing numerous experiments with gases by measuring the amounts of gass that actually reacted. They found that two volumes of hydrogen reacted with one volume of oxygen to form two volumes of water, and that one volume of hydrogen gas reacted with one volume of chlorine gas to form two volumes of hydrogen chloride gas.
![]()
![]()
In 1811, Avogadro proposed the following law:
“Equal volumes of ideal or perfect gases, at the same temperature and pressure, contain the same number of particles, or molecules.”
This Law is later confirmed experimentally. With the basis of Avogadro’s Laws, it became possible to compare the relative weights of various melecules and atoms.
According to Avogadro’s Law:
![]()
n: Number of moles, V: Volume, T: Temperature (Constant), P: Pressure (Constant)
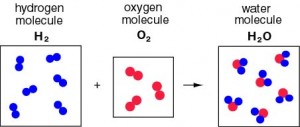
Example: The reaction in which hydrogen and oxygen combine to form water can be displayed as the following.
Avogadro’s Constant
The number of molecules in one mole, that is the number of atoms in exactly 12 grams of carbon-12.
![]()
Sources:
1. Molecules History
2. Avogadro’s Law
3. Introduction of the Concept of the Molecules
One response to “Concept of the Molecule”
[…] The development of this theory in the 19th century are mostly based on the theory of atoms & molecules. Since there are no real experiments during that time, many leading physicists strongly opposed the […]