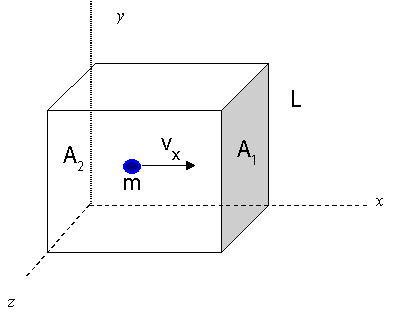
To begin, let's visualize a rectangular box with length L, areas of ends A1 and A2. There is a single molecule with speed vx traveling left and right to the end of the box by colliding with the end walls.
Time between collisions with the wall:
t=vx2L Frequency of collisions:
f=t1=2L/vx1=2Lvx Force according to Newton:
F=dtdp=ma Change in momentum:
Δp=mvx−(−mvx)=2mvx Average force in terms of particle velocity:
F=Δp(f)=2mv(2Lvx)=Lmvx2 Pressure for a single molecule:
P1Molecule=AF=(Lmvx2)/A=LAmvx2=Vmvx2 For N molecules traveling on x-axis:
PNMolecules=Vm(vx12+vx22+vx32....+vxN2) =a=0∑NVmvxa2 Using mean square speed:
PNParticles=VNmvx2 Considering all three dimensions:
v2=vx2+vy2+vz2 With equal distribution in all directions:
vx2=vy2=vz2 Final pressure equation in 3D:
P=3VNmv2 Relating kinetic energy to temperature:
Ekinetic=2mv2∝T Solving for kinetic energy:
mv2=2Ekinetic⇒3mv2=32Ekinetic Introducing Boltzmann constant k:
kT=3mv2=32Ekinetic Combined pressure equation:
P=VN3mv2=VN32Ekinetic=VNkT=VNkT Converting to moles using Avogadro's number:
n=NaN Final ideal gas law:
P=VnRT⇒PV=nRT
Calculation of Constants
One mole of gas in a 22.4L vessel at 273K exerts a pressure of 1.00 atmosphere:
R=nTPV =(1mol)(273K)(1atm)(22.4L) =0.082molKLatm k=NaR k=6.0221x1023/mol0.082Latm/molK =1.3806504x10−25KLatm