Concept of Molecules
Although the atomic theory proposed by John Dalton created a basic structure of the atom, the general idea of molecules was not cleared. In 1809, French chemist Joseph-Louis Gay-Lussac and others began doing numerous experiments with gases by measuring the amounts of gas that actually reacted.
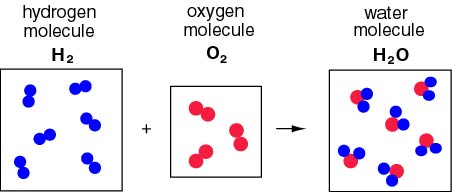
They found that two volumes of hydrogen reacted with one volume of oxygen to form two volumes of water, and that one volume of hydrogen gas reacted with one volume of chlorine gas to form two volumes of hydrogen chloride gas.
Avogadro's Law (1811)
"Equal volumes of ideal or perfect gases, at the same temperature and pressure, contain the same number of particles, or molecules."
This Law was later confirmed experimentally. With the basis of Avogadro's Laws, it became possible to compare the relative weights of various molecules and atoms.
According to Avogadro's Law:
Where:
- n: Number of moles
- V: Volume
- T: Temperature (Constant)
- P: Pressure (Constant)
Water Molecules Formation
The reaction in which hydrogen and oxygen combine to form water demonstrates Avogadro's Law in action.
Avogadro's Constant
The number of molecules in one mole, that is the number of atoms in exactly 12 grams of carbon-12.