18th Century Developments in Atomic Theory

Recreation of Lavoisier's Laboratory Setup
Lavoisier's Contributions
In the 18th Century, significant progress was made in understanding atomic theory through experimental evidence. Antoine Lavoisier, often called the father of modern chemistry, gathered the first scientific data on atoms through quantitative measurements of chemical reactions.
Through his meticulous experiments, Lavoisier discovered that certain substances could not be broken down into simpler components through chemical methods. He defined these fundamental substances as "chemical elements," laying the groundwork for modern atomic theory.
Key Developments
Quantitative Measurements in Chemical Reactions

Lavoisier's Precision Balance for Measuring Chemical Reactions
Lavoisier revolutionized chemistry by introducing precise measurement techniques and the use of the balance. He conducted careful weighing before and after reactions, which allowed him to track the quantities of substances involved. This quantitative approach replaced the qualitative, observational methods that had dominated chemistry for centuries.
Chemical Elements as Fundamental Substances
Through systematic experimentation, Lavoisier identified and categorized substances that could not be broken down further by chemical means. He recognized 33 elements, including oxygen, nitrogen, hydrogen, phosphorus, mercury, zinc, and sulfur. This work laid the foundation for the modern periodic table and challenged the ancient Greek concept of four elements (earth, air, fire, and water).
Development of Systematic Chemical Nomenclature
In collaboration with other chemists, Lavoisier developed a logical naming system for chemical compounds that reflected their composition. This system, published in "Method of Chemical Nomenclature" (1787), replaced the confusing and often mystical names used by alchemists. Many of the principles he established are still used in modern chemical nomenclature, such as:
- Names indicating the elements present in compounds
- Suffixes denoting the proportion of oxygen (-ate, -ite)
- Systematic naming of acids and their salts
Law of Conservation of Mass
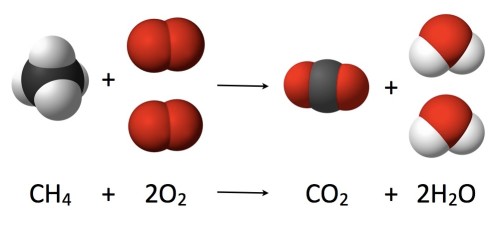
Visual representation of mass conservation in chemical reactions
Perhaps Lavoisier's most significant contribution was the experimental demonstration of mass conservation. Through careful measurements, he showed that in a closed system, the total mass remains constant before and after a chemical reaction. This fundamental principle can be expressed as:
"In nature, nothing is created, nothing is lost, everything changes."
This law became a cornerstone of modern chemistry and provided crucial evidence for the atomic theory of matter. It demonstrated that chemical reactions involve the rearrangement of elements rather than their creation or destruction.
Historical Impact
These developments marked the transition of chemistry from an art to a science. Lavoisier's methodical approach and emphasis on measurement set the standard for modern scientific investigation, while his classification of elements and naming system provided the language needed for clear scientific communication.